Malfunctions or design flaws with continuous blood glucose monitors, insulin pumps, and other equipment can be devastating
By Rachel Rabkin PeachmanPamela, a 64-year-old from Arkansas, began using an insulin pump to manage her type 2 diabetes in 2013. She and her husband, Gary, were trained to use the pump, made by Medtronic, and according to a lawsuit later filed against the company, Gary often helped set up his wife’s device. (CR isn't using their last name to protect their privacy.)
So it wasn't unusual when one night in January 2016, Gary filled the pump’s reservoir with insulin and changed its infusion set, which connects the reservoir to the body via a thin plastic tube.
But Pamela and Gary didn’t know that the infusion set had a flaw that could cause the pump to deliver too much insulin—something that the lawsuit alleged happened to Pamela that night. While Gary was sleeping, Pamela’s blood sugar levels plummeted, and the next day he found her in a coma. Attempts to revive her failed; she died in a hospital a week later.
According to court documents, when Gary obtained readings from his wife’s pump, she had “received in an 18-hour period a dose of insulin that should have been delivered over 5 to 6 days.”
Unlike Pamela and Gary, Medtronic knew about the infusion set’s risk. The company claimed that the situation stemmed from user error. But in June 2013, Medtronic had issued an Urgent Medical Device Safety Notification, two months before Pamela started using her pump, saying that the problem “may cause loss of consciousness or death.”
More on DiabetesWhy Diabetes + COVID-19 Is So Dangerous More From Consumer Reports More From Consumer Reports 5 Diabetes Diet Myths, Busted More From Consumer ReportsBut the lawsuit claims that Medtronic didn't notify Pamela of that possibility when she bought her device. And the Food and Drug Administration, which regulates medical devices, issued no public statement to follow up on Medtronic’s letter. In an internal memo in July 2013, the FDA concluded that it wasn't needed because Medtronic “appears to have properly informed the public.”
Kevin Haverty, a partner at the law firm Williams Cedar in Philadelphia who represented Gary, says the case was resolved out of court in 2018, but he couldn't provide details. Medtronic also didn't comment on the case but told CR that “patient safety is [the company’s] highest priority.”
Pamela’s experience isn't an anomaly. Although diabetes devices, which are used by people with type 1 and type 2 diabetes, have advanced substantially over the past 10 to 15 years and made it easier for them to control their blood sugar, design flaws and malfunctions can have deadly consequences. In fact, diabetes devices account for more adverse event reports—including malfunctions, injuries, and deaths—than any other category of medical device submitted to the FDA, according to a Consumer Reports investigation.
“We’re not tracking device stability and accuracy. We’re tracking people who die or almost die because of inaccurate devices.”
Anna McCollisterHealth Technology Consultant
The FDA told CR that 25 million to 30 million Americans use diabetes devices, and the large numbers of reported incidents reflect “this high volume of use.”
But Madris Kinard, a former FDA analyst who now runs Device Events, a company that aggregates adverse event reports submitted to an FDA database called MAUDE (Manufacturer and User Facility Device Experience), says that the large number of people using the devices can’t fully explain why “these numbers outdistance any other device by far.”
Hundreds of Deaths
To assess what may be driving the high number of adverse events connected to diabetes devices, CR analyzed recent reports submitted to MAUDE, using Device Events. We found that from January 2019 to July 2020, almost 400 deaths and 66,000 injuries were linked to commonly used diabetes devices, such as glucose monitors, glucose test strips, insulin pumps, and infusion sets.
Diabetes devices from multiple manufacturers, including Abbott Laboratories, Dexcom, Medtronic, and Tandem Diabetes Care, were cited in the reports, but no one product stood out.
Instead, what emerged from the sea of product codes was evidence that each diabetes device has its own design intricacies and potential for error, which is then compounded by the fact that they're typically used in conjunction with several other diabetes devices. And they’re all used to manage an already complicated disease that requires constant user involvement.
“It’s not as simple to isolate the problematic device when you’ve got moving parts with insulin, and glucose test strips, and an infusion set,” Kinard says. “It’s so easy, when you’re using more than one device, to blame the other device, or to blame the user.”
Adverse event reports, which can be made by consumers, healthcare providers, or manufacturers, can’t on their own prove that a device caused an injury or a death. MAUDE, which is meant to alert the FDA and manufacturers to potential red flags, offers valuable information. But it's notoriously unwieldy to navigate and has many inconsistencies. And CR found that reports to the database can languish for years without being fully addressed by the government or manufacturers.
“The system is not set up to manage the reporting of all the issues and problems,” says Anna McCollister, a health technology consultant who is a member of two FDA diabetes advisory committees. “We’re not tracking device stability and accuracy. We’re tracking people who die or almost die because of inaccurate devices.”
Dangerous Delays
Take the Medtronic infusion set linked to Pamela’s death. Between June 2013 and May 2014, Medtronic received 750 complaints related to the same infusion sets, according to a deposition referenced in the Arkansas family’s lawsuit. But in January 2016, when Pamela died following the insulin overdose, the problem had still not been corrected.
Further, the lawsuit alleges that it took Medtronic until August 2016 to obtain FDA clearance for a redesign, and that it didn’t start manufacturing the redesigned products until January 2017, after the stock of the older infusion sets were depleted. And though the new, safer infusion sets became available in April 2017, it wasn't until that September—more than four years after the hazard was identified—that Medtronic recalled all infusion sets made before the redesign. It was the September 2017 recall that ultimately instructed consumers to stop using the older models, including the one Pamela used the night of her death.
When asked about these infusion sets and their subsequent recall, Medtronic told CR that it has “rigorous quality systems and reporting standards based on FDA and global regulatory requirements.” The company added that it investigates all reported problems and performs “a risk assessment to determine whether further action is appropriate.”
CR found other examples of both the FDA and manufacturers appearing to be slow to respond to reported problems.
For instance, there was a 57-year-old Texas man with type 1 diabetes who checked his blood sugar regularly using his OmniPod system, which consisted of an insulin pump, made by Insulet, and a FreeStyle glucose meter with test strips, both made by Abbott.
A lawsuit filed against Insulet and Abbott alleges that one day in March 2012, the man’s meter displayed inaccurate glucose readings, preventing him from keeping his actual glucose levels in check before he got into his car. Court documents state that while driving on a Dallas highway, he suffered from a “life-threatening blood glucose level” that “severely impaired his ability to safely operate his vehicle. As a result, [he] lost control of his vehicle, struck another vehicle, and was killed.”
It wasn’t until two years later that his wife received a recall notice from Abbott alerting users to immediately stop using the test strips and the glucose meters because they had the potential to cause erroneous blood glucose results.
They were not the only people allegedly harmed by the defective products while the FDA and manufacturers assessed the risk and investigated the potential hazard. In the two years between the man’s March 2012 death and the February 2014 recall, the MAUDE database shows that numerous adverse event reports were linked to these products, including emergency hospitalizations and at least one additional death.
When asked about the products and the recall, Abbott told CR that “the health and safety of our customers are our highest priority” and that the company’s quality control system “is regularly audited by the FDA.”
Another example of a devastating delay involves the diabetes device maker Dexcom. According to allegations in a lawsuit filed against the company, Dexcom knew of malfunctions and injuries connected to its G4 Platinum continuous glucose monitor as early as 2013, yet took more than two years to fix the problem.
The FDA knew about the problems as well. After conducting a government inspection of a Dexcom facility in November 2013, the FDA sent the company a warning letter in March 2014 citing several issues concerning its G4 monitor. The letter also cited the company’s failures to adequately report adverse events tied to the device. The FDA specified, in part, that Dexcom’s “procedure does not establish internal systems that provide for timely and effective identification, communication, and evaluation of events that may be subject to [medical device reporting] requirements.”
Despite this official warning, it took until February 2016 for Dexcom to issue a recall of the G4 monitor, which could fail to audibly alert users of blood glucose highs and lows, and “could result in serious adverse consequences, including death.” Dexcom’s sluggish response had tragic repercussions: In the year after the FDA sent the warning letter, there were at least 10 deaths and 409 serious injuries reported to MAUDE concerning the G4 monitor.
One accident that took place in that time frame involved a North Carolina man who, in August 2015, wasn't alerted by his Dexcom G4 monitor that his blood sugar was low, according to the aforementioned lawsuit against the company. He “passed out while driving after suffering a dangerously low blood sugar,” court documents state. The man totaled his car and suffered injuries.
When asked about the lawsuit, the warning letter, and the product recall, Dexcom declined to comment.
A Real-Time Tracking Fix
Kinard, the former FDA official, suggests that the FDA’s slow response time and lax enforcement may stem in part from an overwhelmed staff. “When I left the FDA six years ago," she says, "there were around 70,000 [device] adverse event reports per month coming in, and now, we’re over 100,000 reports per month.”
Kinard remembers that the staff wanted “to read every report, but I also know that if they don’t have the time, what they do is they look at deaths, injuries, and then malfunctions. And the next day they come in, and there are more deaths at the top. So those malfunctions keep getting pushed down in the queue.”
One of the many downfalls of this approach is that even if reports of device malfunctions don’t have dire outcomes immediately, they could in the future. For people who depend on the accuracy of their diabetes devices to manage glucose levels through every bite, every workout, and every nap, using a potentially defective product is like taking a gamble on their life over and over again. When the FDA is unable to thoroughly address adverse event reports that concern device malfunctions, it is potentially missing canaries in the coal mine.
The FDA told CR that the agency takes these reports seriously and that MAUDE is “just one important post-market surveillance tool.”
But technology can now track incidents in real time, McCollister says, pointing to the ability of some diabetes devices to aggregate data and communicate electronically with patients and providers.
In fact, patient advocates are championing the development of real-time tracking systems using much of the technology that already exists for these devices. “Congress would need to fund it, but I don’t think we should pretend that we’re doing real adverse-event tracking and reporting, because we’re not,” she says.
The FDA says it supports using new interconnected technologies to track device performance more efficiently. And McCollister notes that FDA officials “have done a very good job honestly of listening to the patient community, taking in what we recommend and what we suggest, and really considering those needs.” But until new tracking systems are implemented, McCollister says the FDA and companies must act more swiftly. “We still have a long way to go.”
How to Protect Yourself
To safeguard yourself and stay on top of malfunctions related to diabetes devices—because even the best ones can be fallible—our experts suggest the following.
Check out the device before you buy it.“I always present all of my demo pumps to each person so they can touch it,” says Alyson Blum, PharmD, a diabetes education specialist at Providence Sacred Heart Medical Center in Spokane, Wash. “There are some pumps that are inherently more intuitive than others,” and there are pros and cons to each.
Beware of inconsistencies with glucose meters.Unlike continuous monitors, which read glucose levels via a sensor under the skin, glucose meters require pricking a finger with a needle and squeezing a drop of blood onto a test strip. While the meters are vital, they're often inconsistent. “We take results with a grain of salt,” says Davida Kruger, a nurse practitioner at the Henry Ford Health System in Detroit.
To improve accuracy, she says, wash and dry your hands before testing, store test strips in a closed vial, and don’t use old strips. She also says that high altitude and temperature or humidity changes can affect readings, as can the user being dehydrated or anemic.
Track recalls and other safety alerts.The FDA and manufacturers address device problems in a variety of ways. For the most severe problems, those that can cause “serious adverse health consequences or death,” consumers may be instructed to send defective products back to the manufacturer. But in other cases, the fix may be a warning along with new instructions on how to use the product.
Regardless of the type of corrective action, it’s critical to be aware of important updates about your devices. To make sure you don’t miss a recall or notification from the FDA, sign up for alerts with the FDA. And be sure the device is registered to your current address.
Investigate problems immediately.If you’re having difficulty with a device, “don’t assume it’s a fluke, or just you or your imagination,” says Diana Zuckerman, president of the National Center for Health Research. Call your healthcare provider or the device manufacturer, which usually has a 24-hour customer service line. This will help you solve the problem and alert the company to potential issues with the device.
Report problems.Tell the FDA and manufacturers about obstacles you encounter. “You have to hold the companies accountable so that they maintain quality control on their product,” Blum says. “It’s frustrating to sit on hold while your device is beeping,” she says, “but I do encourage patients to sit through that call.” To report adverse events directly to the FDA, use MedWatch.
Diabetes Devices ExplainedBlood glucose meters: These devices, introduced in the 1970s, measure how much sugar, or glucose, is in your blood. To use one, you prick your finger with a small needle, squeeze a drop of blood onto a glucose test strip, and then insert the test strip into the glucose meter for its reading. People with both type 1 and type 2 diabetes may need to check their sugar levels several times a day, particularly around meal times, exercise sessions, and before and after sleeping.
Continuous glucose monitors: These devices (also called CGMs), which entered the market a little more than 20 years ago, can be used by people who have either type 1 or type 2 diabetes. As the name suggests, these devices, which are attached to the body, offer glucose readings continuously. This eliminates the need for people to use a finger-prick system several times a day, which is cumbersome and offers only a snapshot of your sugar level at the moment you take the test. One part of the CGM has a tiny sensor, which is inserted under the skin and held in place on the body by an adhesive so that it can continually read glucose levels. A small transmitter on the sensor sends the glucose readings to a separate receiver (in some cases, a smartphone app), where users can view their glucose levels. CGMs need to be removed and replaced, on average, every six to 10 days, depending on the brand.
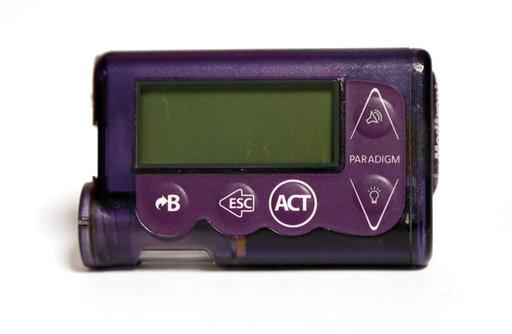
Insulin pumps: These computerized battery-operated devices, used by many people who require insulin to manage their diabetes, are attached to the body constantly and deliver insulin continuously (as opposed to people having to dose their insulin periodically via a syringe). The pumps, which tend to be slightly smaller than a deck of cards, can be worn on a belt or in an armband or a bra, or put in a pocket. They typically deliver insulin through a thin plastic tube that’s connected to a small cannula inserted under the skin. Working with a healthcare provider, consumers can program the pump to deliver small doses of insulin throughout the day and night, and they can administer larger insulin doses, as needed. Some pumps can now “talk” with continuous glucose monitors and receive dosing instructions from the monitors directly.
Infusion set: This component of an insulin pump connects the pump’s insulin reservoir (which holds the insulin supply) to the consumer’s body via a thin plastic tube. When the pump is activated, insulin flows from the reservoir through the tubing and into the patient via a small cannula inserted under the skin.
Editor’s Note:A version of this article also appeared in the January 2021 issue of Consumer Reports magazine.
Rachel Rabkin Peachman
I'm a science journalist turned investigative reporter on CR's Special Projects team. My job is to shed light on issues affecting people's health, safety, and well-being. I've dug deep into problems such as dangerous doctors, deadly children's products, and contamination in our food supply. Got a tip? Follow me on Twitter (@RachelPeachman).
