Introduction
Cardiometabolic disease describes a group of non-communicable diseases, comprising of cardiovascular disease, hypertension, diabetes, metabolic syndrome and chronic kidney disease.1The global burden of cardiometabolic disease has risen significantly in recent years, with cardiovascular disease being the main contributor of premature mortality in non-high-income countries and premature mortality due to diabetes increasing since 2010.2
China has experienced a marked increase in the prevalence of cardiometabolic disease, with the prevalence of diabetes rising from 3.7% in 1990 to 6.6% in 2016.3According to the International Diabetes Federation (IDF), China had the largest number of adult population with diabetes in 2019, and is anticipated to remain so until 2045.4This increase corresponded into a rise in China’s health expenditure, with the highest annual medical expenses attributed to diabetes mellitus, followed by cardiovascular disease.5This heightens the need for effective prevention strategies acting at the risk factor level.
Serum phosphate has been widely associated with cardiometabolic disease and mortality across a number of populations.6–8Correlations have been identified between serum phosphate and cardiometabolic risk factors, including central adiposity,9dyslipidaemia,10hypertension,11and insulin sensitivity.7,12,13Recent studies further propose that high phosphate concentration within the normal range can contribute to cardiometabolic disease through mechanisms such as endothelial thickening and vascular calcification.14,15However, studies on the Chinese population is limited. Given that metabolic diseases such as diabetes mellitus are known to develop at lower body mass indexes in the Chinese population compared to European populations, studies on the Chinese population are required to confirm current findings from European cohorts.16Furthermore, sex-specific differences in the association between serum phosphate and cardiometabolic disease remains under-investigated. This is important as sex hormones may alter phosphate metabolism and cardiometabolic disease development.17Further longitudinal studies are required to bridge this gap in knowledge.
This study tried to fill in the research gap by investigating a large cohort of community-based older Chinese people from Shanghai and followed them over a period of 4 years to examine the prognostic significance of serum phosphate levels on incident cardiometabolic diseases, including heart failure, ischemic heart disease, hypertension, type-2 diabetes mellitus, metabolic syndrome, and chronic kidney disease. It is hypothesized that increased baseline phosphate will predict cardiometabolic disease development.
Materials and Methods
The study was conducted at the Tongji Medical School affiliated Shanghai East Hospital in China, from the year 2013 to 2017. It involved a community-based cohort of older individuals from the town of Gaohang, located north-east within the Pudong district of Shanghai city, along the eastern coast of China. Permanent residents of the Gaohang community were invited to participate in the study, resulting in an initial cohort of 5000 individuals. The exclusion criteria included one or more pre-existing cardiometabolic disease at baseline, and missing or invalid data. The final cohort after 2017 included 3027 participants (1290 men and 1737 women), accounting for a retention rate of 60.5%. Those who were lost to follow-up did not show significant differences in demographic characteristics.
The study protocol was approved by the Institutional Review Board of Tongji Medical School affiliated Shanghai East Hospital. Written informed consent was obtained from all participants. All participants were informed about the purpose of the study, in accordance with the Declaration of Helsinki.
Participants attended the Gaohang community medical centre for an index health screen in 2013. All participants arrived in the morning following an overnight fast of at least 10 hours. Blood samples were obtained upon arrival and analyzed at the Tongji Medical School affiliated Shanghai East Hospital within 2 hours. A double-blind approach was taken by the laboratory technicians, and biochemical analyses were performed using standardized automated analyzers. Relevant reference values were determined using the Chinese Health Industry Guideline.18
Information on past medical history, demographic factors and health-related behaviors were collected by trained family doctors using a standardized questionnaire. Smoking status was categorized into non-smoker, current-smoker, or ex-smoker, and likewise with alcohol drinking. Current-smoker was defined as consuming at least one cigarette daily for at least one year. Ex-smoker was defined as previously satisfying the definition of current-smoker, but has ceased at the time of the questionnaire. Current-drinker was defined as average daily intake of at least 50g alcohol for more than a year, and the category encompassed low, moderate, and heavy drinking. Exercise status was categorized into sedentary, light-intensity exercise, and moderate-intensity exercise, defined using the Physical Exercise guideline.19
Anthropometric measurements including blood pressure, weight, height, and waist circumference were obtained. Three measurements of sitting blood pressure were taken using a mercury sphygmomanometer after 5 minutes of rest. The average of three measurements was recorded. The above procedures were repeated at the 2014 and 2017 follow-up visits.
Heart failure, ischemic heart disease and chronic kidney disease were defined using codes I50, I20-I25 and N18.2-N18.5 from the tenth edition of the International Classification of Diseases (ICD-10) and relevant hospital records. Hypertension was defined as systolic blood pressure ≥130 mmHg and/or diastolic pressure ≥80 mmHg, as per the 2017 American Heart Association Guideline.20Diabetes mellitus was defined as fasting plasma glucose >7.0 mmol/L, based on recommendations by the Chinese Diabetes Society.21Metabolic syndrome was defined using the revised National Cholesterol Education Program Adult Treatment panel III (NCEP ATP III) criteria, where clinical diagnosis of metabolic syndrome requires 3 of 5 constitute diagnosis of: waist circumference ≥90 cm in Asian men and ≥80 cm in Asian women; triglyceride ≥1.7 mmol/L or on medication for elevated triglycerides; HDL <1.03 mmol/L in men and <1.3 mmol/L in women or on medication for reduced HDL; systolic blood pressure ≥130 mmHg or diastolic pressure ≥85 mmHg or on antihypertensive drug; fasting glucose ≥100 mg/dL or on medication for elevated glucose.22
Statistical analysis was performed using SPSS®26.0 (SPSS, Chicago, IL, USA). Continuous variables were presented as mean and standard deviation, while categorical variables were presented as number and percentage.
Baseline inorganic phosphate was categorized into sex-specific quartiles to account for the effect of sex on serum phosphate. Baseline biochemical variables, including BMI, waist circumference, SBP, creatinine, total calcium, bicarbonate, fasting glucose, HbA1c, total cholesterol (TC), triglyceride (TG), and low-density lipoprotein (LDL), were compared against inorganic phosphate quartiles using the ANOVA analysis. The Kruskal–Wallis H-test was used for non-parametric variables. Demographical characteristics, including occupation, education, income, disease history, smoking status and alcohol status were compared against inorganic phosphate quartiles using the χ2test. Variables with statistically significant difference across phosphate quartiles were included in subsequent multivariable analyses as potential confounders.
Cox proportional hazards regression analysis was used to identify the association between sex-specific inorganic phosphate quartiles and incidence of cardiometabolic disease. Significant associations will be investigated further using the machine learning analysis. Statistical significance was set at P<0.05 for all analyses.
Partition analysis was performed using JMP®16 (SAS Institute Inc., Cary, NC, USA). Recursive partitions, or splits, were performed to divide the study population into subgroups based on the optimal outcome predictors. The optimal number of splits was determined based on the Akaike’s Information Criterion score (AICs), with the smallest AICs selected. The R2, RASE, column contribution and prediction profiles were reported.
A second model was performed using the bootstrap forest method. This method combines the predictions from multiple trees together to provide an averaging model. For each outcome, 100 smaller trees were performed, with a minimum of 5 splits and maximum of 10 splits. The best decision tree was selected based on having the lowest Out-of-Bag (OOB) loss. The R2, RASE, column contribution and prediction profiles are reported. All models were performed with 20% validation.
Results
The study population at baseline consisted of 1743 (44.1%) men and 2205 (55.8%) women. Most participants were aged between 66 and 70 years (32.4%), worked as farmers (59.7%), and earned below 1500 CNY (36.4%). Only 4.6% has an education background of college or above. The prevalence of positive disease history is low. Kidney disease history was the most prevalent (6.3%) followed by liver disease (1.8%). The majority did not smoke or drink alcohol (98.7% and 98.6% respectively).
Baseline demographical variables associated with inorganic phosphate quartiles are presented in Table 1. Participants were classified into four sex-specific quartiles using baseline inorganic phosphate concentration. For men, the lowest quartile included values below 0.91 mmol/L (n=455), the second quartile included values between 0.91 and 1.01 mmol/L (n=466), the third quartile included values between 1.01 and 1.11 mmol/L (n=425), and the highest quartile included values above 1.11 mmol/L (n=397). For women, the lowest quartile included values below 1.07 mmol/L (n=601), the second quartile included values between 1.07 and 1.16 mmol/L (n=562), the third quartile included values between 1.16 and 1.25 mmol/L (n=523), and the highest quartile included values above 1.25 mmol/L (n=519).
Table 1 Baseline Demography Based on Inorganic Phosphate Quartiles |
Among men, smoking status showed significant difference across inorganic phosphate quartiles. The highest quartile showed the greatest proportion of current smokers (35.8%).
Among women, baseline inorganic phosphate was significantly associated with education (p=0.048) and history of kidney disease (p=0.001). Educational attainment equivalent to primary or below primary education was more prevalent in participants with lower quartiles (29.5% and 25.7%) compared to higher quartiles of inorganic phosphate (23.4% and 21.4%). History of kidney disease was more prevalent in the highest quartile (44.4%). No significant difference was observed for occupation, income, other disease histories, smoking status and alcohol status.
Baseline biochemical variables associated with inorganic phosphate quartile are presented in Table 2. For men, statistically significant differences were observed in SBP (p=0.004), total calcium (p<0.001), bicarbonate (p<0.001), fasting glucose (p<0.001), HbA1c (p<0.001), and TC (p=0.032) across inorganic phosphate quartiles. Compared to men with lower phosphate quartiles, the highest quartile was associated with lower SBP (137.23±16.71mmHg), greater total calcium (2.312±0.108mmol/L), lower bicarbonate (26.375±2.937mmol/L), greater fasting glucose and HbA1c (5.927±2.059mmol/L and 6.309±1.281%), and greater total cholesterol (4.783±0.918mmol/L).
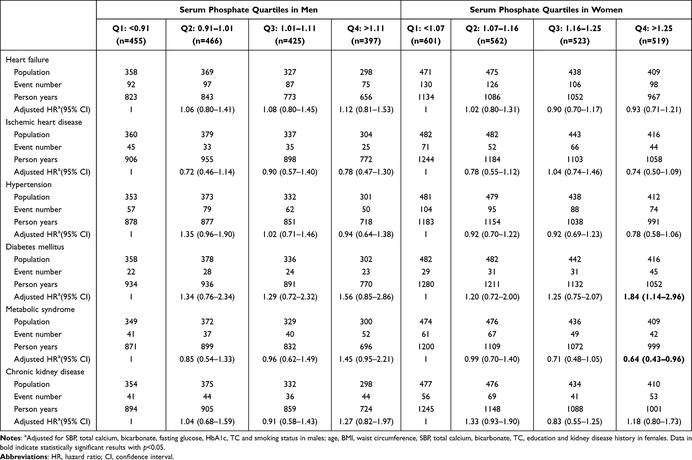
Table 2 Biochemical Variables Associated with Inorganic Phosphate Quartiles |
Among women, there were significant differences between inorganic phosphate quartiles and age (p<0.001), BMI (p=0.035), waist circumference (p=0.001), SBP (p<0.001), total calcium (p<0.001), bicarbonate (p=0.005), and TC (p=0.022). Compared to lower quartiles, the highest quartile of inorganic phosphate was associated with younger age (71.24±6.454 years), lower BMI (24.569±3.553kg/m2), lower SBP (136.84±17.503mmHg), higher total calcium (2.330±0.112mmol/L), lower bicarbonate (26.605±3.040mmol/L), and higher TC (5.248±0.973mmol/L). No significant associations were observed between inorganic phosphate and variables such as creatinine, fasting glucose, HbA1c, TG, and LDL (all p>0.05).
During the follow-up period, 1856 of the 3027 subjects developed at least one cardiometabolic outcome (61.3%). In total, there were 811 cases of incident heart failure (25.8%), 371 cases ischemic heart disease (11.6%), 609 cases of hypertension (19.2%), 233 cases of diabetes mellitus (7.3%), 389 cases of metabolic syndrome (12.4%), and 384 cases of chronic kidney disease (12.2%).
Adjusted hazard ratios with 95% confidence interval are shown in Table 3. For both sexes, the lower quartiles (≤1.20 mmol/L) were used as the reference group. The model was adjusted for SBP, total calcium, bicarbonate, fasting glucose, HbA1c, TC and smoking status in men, and age, BMI, waist circumference, SBP, total calcium, bicarbonate, TC, education and kidney disease history in women.
Table 3 Risk of Cardiometabolic Diseases by Baseline Phosphate Quartiles in Men and Women |
When compared using phosphate quartiles, there was a statistically significant difference in the incidence of diabetes mellitus and metabolic syndrome among women (p=0.013 and p=0.030 respectively). Women with serum phosphate greater than 1.25 mmol/L were 1.835 times more likely to develop diabetes mellitus compared to women with levels less than 1.07 mmol/L (95% CI 1.139–2.957). The risk of developing metabolic syndrome is 0.360 times lower in women with the highest level of inorganic phosphate compared to the lowest quartile (95% CI 0.428–0.957).
For men, higher phosphate quartiles were associated with increased risk of developing diabetes and metabolic syndrome. Compared to men in the lowest phosphate quartile, men with phosphate greater than 1.11 mmol/L were 1.453 times more likely to develop metabolic syndrome (95% CI 0.954–2.213). Similarly, the risk of developing metabolic syndrome was 1.561 times greater in the highest quartile (95% CI 0.853–2.856). However, these associations were marginal (p=0.082 and p=0.149).
Partition analysis and bootstrap forest analysis were performed for diabetes mellitus and metabolic syndrome development in women. Confounding variables identified in the bivariate analysis are included in the models. Fasting glucose was removed for diabetes mellitus as it was part of the diagnostic criteria. Similarly, waist circumference, SBP, HbA1c, TC and TG were not included as they define metabolic syndrome.
For diabetes mellitus, the bootstrap forest model explained 12.2% of the variance observed in disease outcome with an estimated error of 0.248, compared to the partition model which explained 6.5% with a higher estimated error of 0.264. For metabolic syndrome, the bootstrap forest model accounted for 16.2% of the observed variance with an estimated error of 0.309, whereas the partition model explained 9.5% of the disease outcome with an estimated error of 0.321. Therefore, the bootstrap forest model was chosen for both outcomes.
The best decision tree for predicting the 4-year probability of developing diabetes mellitus was selected using the smallest out-of-bag (OOB) loss. It used seven variables (inorganic phosphate, waist circumference, bicarbonate, BMI, TC, education status, and total calcium) to perform 19 splits. The overall probability of developing diabetes mellitus in all female participants was 7.96%. The highest quartile of inorganic phosphate (>1.25mmol/L) was identified as the most significant predictor, with a 4-year risk of 14.54%.
For metabolic syndrome, the overall probability of developing metabolic syndrome in all female participants was 14.8%. A total of 19 splits were performed using BMI, total calcium, bicarbonate, kidney disease history, age, occupation, inorganic phosphate, education, and alcohol use as predictors. The most significant predictor of metabolic syndrome was BMI.
Table 4 shows the decision rules and subgroups, or terminal nodes, identified by the decision tree. High inorganic phosphate (>1.25mmol/L) was the most significant predictor of 4-year diabetes mellitus development. In females with high inorganic phosphate (>1.25mmol/L), bicarbonate was the next predictor. The probabilities of developing diabetes mellitus in females with high inorganic phosphate who has bicarbonate ≥24.1mmol/L and <24.1mmol/L were 12.6% and 24.1% respectively. Among females with lower quartiles of inorganic phosphate, those with waist circumference greater than 86cm, and TC <3.14mmol/L had the highest probability of developing diabetes mellitus (50.0%).
Table 4 Rules Identified by Decision Tree to Determine Metabolic Disease Outcomes |
For metabolic syndrome, the greatest risk was associated with having a BMI <23.81kg/m2 (24.6%). The next best predictor was total calcium. The highest risk was estimated in females with BMI <23.81kg/m2, other occupations, and total calcium 2.25–2.33mmol/L (66.7%).
As shown in Table 5, inorganic phosphate quartiles contributed 9.2% to the variance explained for diabetes mellitus. BMI was the greatest predictor, accounting for 24.0%, followed by TC (19.9%), total calcium (11.3%), waist circumference (10.1%), bicarbonate (8.5%), education (7.1%), and SBP (6.9%). Age and history of kidney disease contributed negligible amounts to the 4-year risk of developing diabetes mellitus (2.2% and 0.7% respectively).
Table 5 Variable Contributions for the Development Metabolic Disease Outcomes |
Inorganic phosphate quartile was not a significant contributor to the development of metabolic syndrome (3.5%). BMI was again the most significant contributor, accounting for 51.1% of the variance explained by the model. The second greatest contributor total calcium (13.8%) and bicarbonate (13.3%), followed by kidney disease history (6.9%). Age (4.7%), occupation (4.6%) did not contribute significantly. Lastly, education and alcohol use had negligible effects on the risks of developing metabolic syndrome (1.3% and 0.8% respectively).
Discussion
The present study demonstrates the importance of inorganic phosphate in the development of type-2 diabetes mellitus among a population of older Chinese women. Serum phosphate greater than 1.25 mmol/L showed an estimated risk of 14.54%, compared to 5.88% observed in lower quartiles. This finding is contrary to previous studies which identified lower serum phosphate in subjects with type-2 diabetes mellitus compared to non-diabetic subjects.23Similarly, lower, rather than higher, serum phosphate has been associated with impaired glucose tolerance and insulin sensitivity.7,12,24,25
However, similar findings have been reported in some population studies. The IRAS (Insulin Resistance Atherosclerosis Study) investigated African-American, Hispanic, and non-Hispanic individuals, with serum phosphate concentration greater than 1.20 mmol/L associated with 2.24 times greater risk of developing diabetes compared to individuals with serum phosphate lower than 1.00 mmol/L, independent of glucose tolerance and insulin parameters.26Similarly, high serum phosphate was associated with increased risk of diabetes mellitus in a population of Taiwanese individuals aged over 60 years (HR 1.49, 95% CI 1.15–1.92).27Such heterogeneity may suggest ethnic- or culture-specific associations. Alternatively, as our cohort consisted of older post-menopausal women, it is possible that increased serum phosphate observed at baseline was reflective of bone health rather than glucose metabolism.
Several mechanisms have been proposed for the interaction between serum phosphate and diabetes development. One mechanism is the involvement of phosphate in regulating energy balance and oxygen consumption.28Phosphate is heavily implicated in both gluconeogenesis and glycolysis through its role in the phosphorylation of carbohydrate intermediates.12Haglin et al hypothesized that higher serum phosphate may be associated with depletion of intracellular phosphate.10Depletion of intracellular phosphate may lead to a lack of substrate for phosphorylation, which inhibits the peripheral utilization of glucose and subsequently results in impaired insulin secretion and glucose tolerance.29,30Studies of phosphate metabolism in diabetic patients have also suggested that higher oxygen consumption was associated with lower concentrations of inorganic phosphate.31
Another mechanism is the positive effects of serum phosphate on insulin secretion and sensitivity. Hypophosphatemia, not hyperphosphatemia, has been associated with impaired insulin sensitivity in both hyperglycemic and euglycemic states.7This is hypothesized to be due to insulin’s capacity to stimulate intracellular phosphate uptake via insulin-dependent transporters and phosphorylation of metabolic intermediates, which exacerbates hypophosphatemia and results in further hyperinsulinaemia.8
Lastly, changes in serum phosphate reflects impaired renal reabsorption. Higher serum phosphate has been associated with increased HOMA-IR in non-diabetic participants with impaired renal function.32Hyperphosphatemia has also been noted in diabetes, especially in the context of diabetic nephropathy.33Therefore, poor renal function may have a mediating effect between phosphate retention and insulin resistance. However, although this study found a significant association between higher serum phosphate and existing kidney disease in women (p<0.001), serum phosphate was not associated with kidney disease development (p=0.411), nor was baseline kidney disease a significant contributor to diabetes (p=0.526). These findings suggest serum phosphate may increase the risk of type-2 diabetes mellitus through an unclear mechanism that is independent of kidney disease.
Although inorganic phosphate above 1.25 mmol/L was significantly associated with lower risks of metabolic syndrome development in the multiple regression model (HR 0.640, 95% CI 0.428–0.957), it had a negligible contribution in the bootstrap model (3.5%). BMI was the greatest contributor (51.1%), which may be explained by its close relation to waist circumference, a diagnostic criterion of metabolic syndrome. Overall, this finding supports previous cohort studies, which identified significant associations between inorganic phosphate and metabolic diseases. Jhuang et al identified a positive association between serum phosphate at baseline and development of metabolic syndrome in those aged above 60 years (HR 1.39, 95% CI 1.11–1.74).27They reported no significant association in those aged below 60, likely due to lower baseline serum phosphate compared to those aged over 60, which is consistent with the findings of this study. However, the sex-specific differences identified in our cohort was not reported. A cross-sectional study performed by Shimodaira et al on the older Japanese population identified contrary sex-specific differences.24Among 16,041 participants (9076 men and 6965 women), low serum phosphate was associated with metabolic syndrome in men, whereas high serum phosphate was associated with metabolic syndrome in women.24In a cohort of middle-aged British men, lower rather than higher baseline serum phosphate was associated with increased components of metabolic syndrome.8The mechanism behind this disparity remains unclear. However, the effect of age cannot be excluded, as our study cohort had a mean age of around 70 years at follow-up, while the latter two studies included participants aged around 50 and 60 years respectively.8,24Moreover, ethnicity and cultural differences may have contributed, as the development of metabolic syndrome varies due to gene-environmental interactions.34Overall, our results suggest that low serum phosphate is significantly associated with risk of metabolic syndrome in older Chinese women.
Low phosphate has been linked to obesity. Obeid argued that reduction in intracellular phosphate availability causes low thermogenesis secondary to reduced ATP production.35Thermogenesis has been shown to affect weight control, with low thermogenesis associated with greater predisposition to gain weight.36This is possibly due to the effects of low thermogenesis on hunger and appetite.37In support of this hypothesis, a randomized controlled trial has demonstrated that phosphorous supplementation reduces body weight, BMI and waist circumference, as well as subjective appetite.38
Furthermore, low serum phosphate may be an indicator of poor diet. Stoian et al suggested that altered phosphate levels may result from changes in intestinal absorption and internal redistribution.39Diets high in carbohydrate and low in protein provide limited dietary phosphate. Low protein and high carbohydrate diets have been associated with three-times greater odds of metabolic syndrome in elderly women, as well as increased central adiposity and dyslipidaemia.40Furthermore, serum phosphate can predict blood pressure elevations. A possible mechanism is its role in promoting sympathoadrenal activity through increasing plasma adrenaline.8Higher serum phosphate has been shown to contribute towards vascular and soft-tissue calcification,14which increases arterial wall stiffness and predisposes to hypertension and cardiovascular disease.41However, the mechanism behind significantly lower blood pressure observed in the women of this study remains unclear.
In women, high serum phosphate was associated with age, BMI, waist circumference, SBP, total calcium, bicarbonate, and total cholesterol. This was largely consistent with previous studies, which reported significant associations between high serum phosphate and lower systolic blood pressure, lower waist circumference.9,15,42,43The associations with lower triglyceride and fasting blood glucose were not significant in our study population. A possible explanation is variation in the distribution of serum phosphate measurements, which resulted in different cut-off values. For example, Park et al used 5.0 mg/dL to define high serum phosphate,42which was considerably higher than the values used by this study. Age differences may also have contributed to this, as previous studies were conducted on younger cohorts aged around 40 years. A recent study by Yoo et al suggested that serum phosphate in women increases until age 60 and decreases thereafter.44This could have accounted for the lower serum phosphate and subsequent lack of association seen in our cohort. The decrease in serum phosphate after age 60 may also explain why our results showed an inverse association with age, contrary to previous studies conducted on younger women.15,44
In men, high serum phosphate at baseline was positively associated with systolic blood pressure, total calcium, bicarbonate, fasting glucose, HbA1c, and total cholesterol. Elevated blood glucose and cholesterol are established risk factors for cardiometabolic disease.45,46Previous studies have reported similar sex-specific differences in lipid profiles, where serum phosphate was positively correlated with total cholesterol and increased cholesterol-to-HDL ratio.47While this study supported previous associations between phosphate in men and altered lipid profiles, the association with greater glucose and HbA1c was contradictory to previous studies. Cohort studies conducted on older Japanese,24Dutch,44and New Zealand9men have observed lower fasting glucose with higher serum phosphate. Differences in baseline characteristics such as age may have contributed to this disparity, as older age has been linked to progressively worse plasma glucose control.48Fat mass has also been suggested to mediate the relationship between serum phosphate and glucose.9As our cohort consisted of leaner rather than obese men by BMI, this may have contributed to the contradictory findings. Lastly, the possibility of this association being unique to Chinese men cannot be ruled out.
Our study expanded on current understandings of serum phosphate and metabolic disease by investigating a population with limited previous insight. Furthermore, we investigated sex-specific differences within this population, which has not been previously reported in the Chinese population. Second, although dietary information was recorded, it did not specify the amount of protein intake. Thus, we could not analyze whether dietary phosphate intake impacted on the findings. Third, although conventional confounding factors were identified and included in the multivariate analysis, some factors such as FGF23 and activated vitamin D were not included in the study. Future study involving large number of participants and long-term follow up will be conducted to confirm the study findings.
Conclusion
In conclusion, baseline serum phosphate greater than 1.25 mmol/L was significantly associated with increased risk of type-2 diabetes mellitus, and decreased risk of metabolic syndrome in older Chinese women. The 4-year risk of developing diabetes mellitus in women with the highest phosphate quartile was 14.54%, with inorganic phosphate contributing to 9.2% of the variance explained by the model. Our results suggest the presence of sex-specific associations between serum phosphate and diabetes mellitus, possibly explained by the difference in risk factor profile. This finding may be important due to its potential prognostic value in assessing metabolic risk among disease-free older individuals. However, further longitudinal studies are required to validate the observed associations.
