When you’ve got a diabetic in your life, there are few moments in any day that are free from thoughts about insulin. Insulin is literally the first coherent thought I have every morning, when I check my daughter’s blood glucose level while she’s still asleep, and the last thought as I turn out the lights, making sure she has enough in her insulin pump to get through the night. And in between, with the constant need to calculate dosing, adjust levels, add corrections for an unexpected snack, or just looking in the fridge and counting up the number of backup vials we have on hand, insulin is a frequent if often unwanted intruder on my thoughts.
And now, as my daughter gets older and seeks like any teenager to become more independent, new thoughts about insulin have started to crop up. Insulin is expensive, and while we have excellent insurance, that can always change in a heartbeat. But even if it does, the insulin must flow — she has no choice in the matter. And so I thought it would be instructional to take a look at how insulin is made on a commercial scale, in the context of a growing movement of biohackers who are looking to build a more distributed system of insulin production. Their goal is to make insulin affordable, and with a vested interest, I want to know if they’ve got any chance of making that goal a reality.
That’s a Lot of Pigs
To understand what’s involved in making artificial insulin, the best place to start is with a look at natural insulin. Insulin is a hormone involved in the regulation of blood glucose levels, secreted by the pancreas. Specialized cells, called beta cells, sense the level of glucose in the blood, which typically spikes after eating a meal, and secrete insulin into the bloodstream in response. Insulin quickly makes its way around the body, interacting with cells by stimulating their glucose transport system to take up blood glucose for their metabolic needs.
In Type 1 diabetes, though, the ability of the pancreas to produce insulin has been destroyed. The reasons for this are unclear, but in general it’s thought to be at least partially related to autoimmunity, where someone’s own immune system recognizes the pancreatic beta cells as foreign cells and destroys them. Type 1 diabetics therefore lack the ability to sense and respond to rising blood glucose either partly or in total, meaning they have to inject insulin regularly to survive. Type 2 diabetes is a completely different disease, caused in part by the body becoming insensitive to insulin. It’s treated with a wide range of other medications, although some Type 2 diabetics take insulin too.
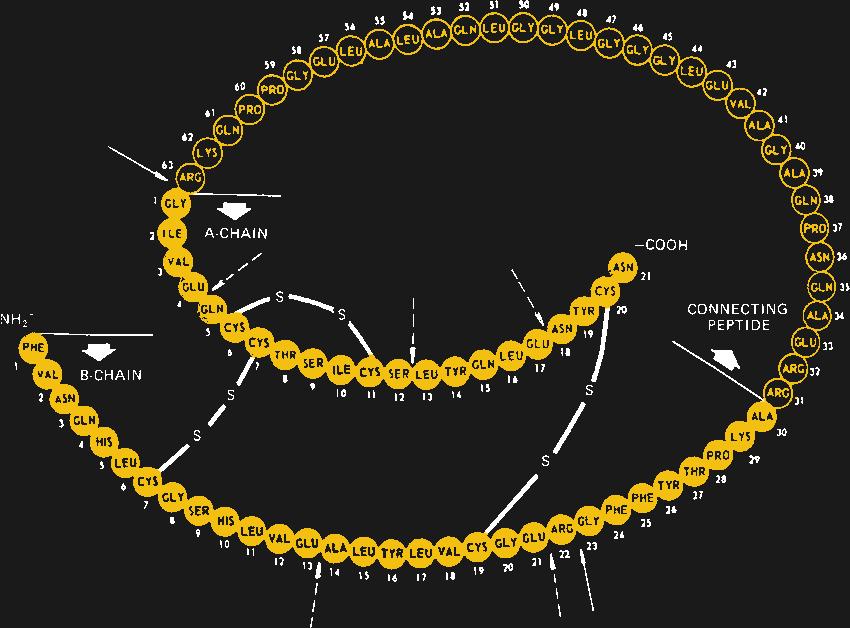
When first identified as a treatment for diabetes in 1920 by Canadian doctors Banting and Best, it came as a surprise to learn that insulin is actually a protein. Up to that point, it had been assumed that all hormones would be small molecules, so the discovery that insulin is actually a protein composed of 51 amino acids in two separate chains that are linked together in three places by disulfide bonds came as quite a shock. It would also open the door to the current recombinant insulins on the market today.
But, for the first 60 years or so of its commercialization, insulin was derived strictly from animal sources, chiefly from the pancreases of pigs. The amino acid sequence of insulin is highly conserved across species, and even insulin derived from fish has a clinical effect in humans. Porcine insulin differs from human insulin by only a single amino acid, but purifying it was a difficult process. It took two tons of slaughterhouse offal to create just 250 ml of insulin, through a thoroughly disgusting process of grinding, mincing, and extracting the insulin, and purifying it to the point where it was free enough from contaminating substances to not raise an immune response when injected. And yet, sometimes it still did.
All that changed in the 1980s with the introduction of recombinant insulin, made in genetically engineered microorganisms. By putting the human insulin gene into a small loop of DNA, called a plasmid, and coaxing those plasmids into bacteria or yeast, cell lines were created that were capable of producing vast amounts of insulin, in a way that was easy to scale up and far easier to purify than from mammalian tissue. It also had the advantage of being identical to natural human insulin, down to the last amino acid. And, it opened the door to tweaking the amino acid sequence with genetic engineering, to produce different kinds of insulin with specific characteristics.
Scaling Up
Today, most insulin is produced in the common bacterium Escherichia coli, although some manufacturers prefer to use the yeast Saccharomyces cerevisiae for their processes. Regardless of the microorganism, the process of producing a batch of insulin is pretty straightforward. Genetically modified cells are used to inoculate a large volume of liquid culture medium in a device called a bioreactor. This is basically a huge stainless steel tank to hold the fermenting culture, along with heaters, mixers, aerators, and an array of sensors to control and monitor the growth process. There are often multiple bioreactors in series, with the output of a smaller one being fed into a larger one, to step up the volume of culture gradually.
The genetically modified microorganisms are allowed to grow until they’ve reached the maximum growth rate, at which point they are harvested from the bioreactor. The first step is filtration, to separate the insulin-filled cells from the spent growth medium. The cells are then split open with a high-pressure homogenizer, leaving behind a mixture of cell debris and insulin inclusion bodies, which are sort of like crystals of the hormone that formed in the cells during fermentation.
This is the point where things get touchy. Up to now, the insulin has been pretty safe inside the cells. Once the cells are cracked open, though, all their proteins, including enzymes that degrade and recycle other proteins (proteases), can start acting on the insulin, degrading it and reducing the yield. Avoiding this is a matter of choosing the right buffers, adding the proper protease inhibitors, and keeping everything at a low temperature. Acting quickly is important too — the longer the insulin is exposed to the cellular debris, the more chance there is for degradation.
After a series of solubilization steps that further separates the insulin from cellular debris, a round of post-processing may need to be performed. In the body, insulin is expressed as a single long chain of amino acids, called proinsulin, which folds back onto itself and forms a tertiary structure between distant cysteine residues. Once that structure is established, the loop is cleaved in two places, leaving the active two-chain hormone. Manufactured insulin needs to replicate this structure; depending on the manufacturer, this may be achieved either by growing the A- and B-chains separately and combining them chemically, or by producing proinsulin and processing it after the fact.
Either way, the almost finished insulin still needs some final purification. This is often accomplished using affinity chromatography, where the insulin produced by the microorganisms is provided with a “tail” of protein. The tail allows the protein to bind to antibodies, which are attached to a solid-phase matrix of some sort. When a solution containing the fusion protein is allowed to flow over the matrix, anything not bearing the protein tail flows right out with waste, leaving the insulin fusion protein stuck to the matrix. The pure fusion product is then freed from the column by tweaking the pH of the solution, cleaved from the protein tail, and given a final purification with reverse-phase chromatography before going through rigorous QC checks and final packaging.
Smaller Might Be Better
While commercial insulin production is certainly complicated and expensive, it’s not because the process itself is difficult. Creating and maintaining transgenic cell lines, expressing fusion proteins, and purifying the end product is something that’s done by grad students in biology labs around the world every day. The complexity of commercial insulin production comes from the degree to which it must be scaled up to be financially viable, leading to huge labs with rows of bioreactors, endless miles of stainless steel piping, and an extremely complicated command and control system to make sure it all works. Comparing a modern biologics plant, which would make not only insulin but other biologic drugs like immunotherapeutics, to a semiconductor fab, is apt: both capture the essence of the problems and the potential for scaling — while it’s entirely possible to make semiconductors in your garage, it’ll never be commercially viable to do so.
Embracing this brutal reality is the key to solving the insulin supply problem, at least in the eyes of the OpenInsulin Foundation. A team of biohackers with a wide geographic footprint, OIF is a non-profit focused on creating community-scale systems that can produce safe, affordable insulin. Over the past six years, their volunteers have been busy building transgenic bacteria, working on culturing processes and equipment, and dealing with the fussy purification steps, and they’ve succeeded in seeing the procedure all the way through to finished product.
Now that OIF has the science figured out, the next step is to scale it appropriately. In a presentation from 2020, Louise Lassalle, the group’s communication manager, gave a breakdown of the equipment needed to make insulin on a medium scale — in bigger batches than are possible on the benchtop, but far, far smaller than what commercial biologics plants are capable of. It’s not cheap — about $1 million worth of gear. But, that investment would produce enough insulin for 14,000 diabetics, meaning that a functioning, community-level insulin factory could conceivably be funded for about $70 per person. And that factory would produce insulin at around $6 a vial, taking into account everything from raw materials to salaries, rent, and utilities.
Granted, there are huge and potentially insurmountable legal and regulatory hurdles with this plan. It may well be that the insulin industry, with a vested interest in keeping prices high — at least in the United States — will let fly their legal dogs of war, and shut this group down if they ever get close to realizing their goal. And they’ll certainly have to deal with the Food and Drug Administration if they ever expect to actually use their insulin on humans. On the other hand, the efforts of the Open Insulin Foundation and other biohackers working on alternative sources of insulin might just win in the long run, by showing that making insulin just isn’t as hard as we’ve perhaps been lead to believe, and that maybe scaling everything up to massive proportions isn’t always in the best interests of the consumer, even if it’s profitable business.
This leads to another, possibly more important point. If the last year and a half have taught us nothing else, it’s that long supply chains need to be looked at with skepticism. When a pharmaceutical company builds a mega factory for financial reasons, it creates a single point of failure that probably doesn’t need to be there. We’ve seen how easily supply lines can be pinched, and it seems incredibly foolish to engineer into a system the potential to shut down the world’s supply of any medicine, especially one as vital as insulin. OIF’s vision of distributed mini-factories for insulin production seems smarter right now, and will probably only seem like a better idea as time goes on and complex systems become less and less reliable.
