Introduction
Diabetes mellitus (DM) is a group of metabolic disorder with various etiologies characterized by chronic high blood glucose levels as results of carbohydrate, fat, and protein metabolism disturbances.1
Globally, above 400 million adults live with DM, a disease, which caused 1.6 million mortality in 2015.2The number of people with DM in the world is estimated to increase from 382 million in 2010, to 592 million in 2035, and one among ten of the world’s population could be affected by diabetes, by the year 2035.3
The T2DM accounts for 90–95% of all DM and affects about 7% of the general population.1T2DM has increased at a rapid rate and becomes a serious health problem globally,1,4and its occurrence is on the rise, especially in middle-income and low-income countries.5
The chronic hyperglycemia of diabetes, especially when poorly controlled, causes long-term damage, dysfunction, and failure of different organs of the body like the eyes, kidneys, nerves, blood, and blood vessels.6Anemia is one of the commonest and prevalent blood-related disorder occurs in patients with diabetes.7It mostly occurs in DM patients who also have renal impairment.8,9Evidence indicates that the existence of anemia among T2DM is typically associated with the failure of the kidney to produce appropriate erythropoietin.10–13The risk of occurring anemia among DM patients with kidney disease is higher and occurs earlier than in those patients with the same level of renal impairment from other etiologies.8,9,14Nevertheless, the early occurrence of anemia in DM patients without renal impairment,15and the occurrence of more frequent and more severe anemia in DM patients compared with patients with the same level of renal impairment from other causes,9,14,15highlights the presence of some other causes of anemia in these patients.15
Diabetic neuropathy, chronic inflammatory activity, increased levels of advanced glycation end products (AGEs), erythropoietin hypo-responsiveness, effects of oxidative stress, and anti-diabetic medications are other possible cause of anemia in DM patients.6,16–20
Results from many studies showed in the diabetic clinics, the prevalence of unrecognized anemia is nearly two to three folds greater than the general population. In addition, DM patients tend to develop anemia at earlier ages and with greater severity than the general population, putting these patients at greater risk of complications, and this additional burden greatly contributes to patient’s co-morbid vascular disease and adverse outcomes.21–26
The prevalence of anemia among T2DM patients varies in different areas; up to 20% in Australia,2746.5% in Caribbean population,2841.7–63% in Pakistan,29,3041.4% in Cameroon,3129.8% in Ethiopia,32and 63% in Egypt.33
Anemia in DM patients is a common and often neglected and untreated complication of diabetes, which may have a negative consequence on the development and progression of other diabetes-related macrovascular and microvascular complications which can further enhance anemia progression, making the vicious cycle.34Growing evidence indicates that anemia in T2DM patients is a strong and independent indicator of increased risk for diabetes-related macrovascular and microvascular complications.10,35–38It causes early occurrence and rapid progression of complications like diabetic nephropathy, diabetic retinopathy, diabetic neuropathy, end-stage renal diseases, ischemic heart disease, and non-healing diabetic foot ulcers.35
As in Ethiopia with an increasing incidence of DM,39it becomes mandatory to be aware of such co-morbidities at the earliest. Despite these facts, information on the prevalence of anemia and its associated factors among DM patients is very few in sub-Saharan African countries, including Ethiopia, where other additional potential contributory factors like nutritional deficiencies as well as infectious diseases are very common and expected to worsen the burden of anemia.
This study will provide important information concerning the burden of anemia and its associated factors among T2DM, used as baseline data for further investigation and will be helpful for policymakers, and other stakeholders to develop interventions that on emphasize on routine screening, and proper management of anemia among T2DM patients. Hence, this study was undertaken to determine the prevalence of anemia and its associated factors among T2DM patients at Debre Berhan Referral Hospital.
Materials and Methods
Hospital-based cross-sectional study was conducted among T2DM patients at DM follow up clinics at Debre Berhan Referral Hospital (DBRH) in North-East Ethiopia, over a period of two months (April 1 to May 30/2019). DBRH is located in Debre Berhan town of Amhara Regional state, about 130 kilometers North-East of Addis Ababa on the main road to Dessie, and has an elevation of 2840 meters above sea level. Currently, DBRH is the only public Hospital in Debre Berhan Town, it serves for 2.8 million catchment population.40There are different units and clinics that provide service for clients. Among these clinics, the diabetes clinic registers, treat and provide care for all diagnosed diabetic patients.
A total of 249 T2DM patients with more than six months’ follow-up at the diabetic clinic were included in the study by using a systematic random sampling technique. All adult T2DM patients (≥18 years) attending the diabetes clinic in the study periods were considered in the study. Patients with known hematological diseases, patients who had a history of delivery within 3 months before the data collection period and, pregnant women, those who were critically ill, and those patients with a history of acute or chronic blood loss and blood transfusion within 3 months of enrollment were excluded. Patients were also excluded if they were known chronic liver disease (CLD) patients, human immunodeficiency virus infection, and malignancy including hematological malignancies.
Sample size was calculated using a single population proportion formula, taking p=29.8% (anticipated proportion of anemia in T2DM),325% tolerable margin of error (d=0.05) and confidence interval (CI) of 95% (Z a/2 =1.96). Then the minimum sample size obtained was 321. A correction formula was employed and became 226. By adding 10% non-response rate, a total of 249 T2DM patients were included in the study. To select the study participants, a systematic random sampling technique (i.e., every third patient) was used.
Data were collected by using semi-structured questionnaire. Three data collectors (one nurse and two laboratory professionals) were collect the data. The collected information includes socio-demographic characteristics, clinical characteristics, anthropometric measurements, and laboratory analysis. Socio-demographic data and clinical characteristics like duration of DM were collected using an interview guide; whereas the presence of diabetes-related complications like; retinopathy, neuropathy, nephropathy, and other complication; history of hypertension and current diabetic medications were collected from reviewing of patient’s medical records. Four consecutive fasting blood glucose measurements, including measurement at the time of the data collection period were also recorded from the patient’s medical records for calculating the mean blood glucose level.
Anthropometric measurements such as weight (kg), height (m), and waist circumference were measured according to WHO recommendations. The body mass index (BMI) was computed as weight in kilograms divided by the square of the height in meters (kg/m2). The BMI of the participants were classified as: underweight less than 18.5 kg/m2, normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥ 30 kg/m2).41Central obesity as measured by waist circumference is defined as >102 cm in males and >88 cm in females.42Blood pressure (BP) was measured using an aneroid sphygmomanometer after 10 mins of rest in a sitting position. Hypertension was defined as Systolic Blood Pressure (SBP) ≥130 mmHg and/or Diastolic Blood Pressure (DBP) ≥80 mmHg or current use of antihypertensive medication.
For laboratory data, from each participant six mL of venous blood was collected under aseptic conditions by venous puncture from the vein using a disposable syringe as follows: 3 mL into ethylene diamine tetraacetate (EDTA) tube for hemoglobin and red blood cell (RBC) indices determination, and the remaining 3 mL into a plain tube for serum creatinine analysis. Hemoglobin (Hgb) values and RBC indices (MCV, MCH, and MCHC) were calculated using the ABX Micros 60 Hematology Analyzer (Horiba-ABX, Montpellier, France). For serum creatinine analysis, the remaining 3 mL of blood was collected in a clot activator with a gel test tube and allowed to clot at room temperature for 30 mins. After complete coagulation, the cells were separated from the serum by centrifugation at 3000 RPM for 5 mins. Then serum creatinine was determined using ECHO XPC automatic chemistry analyzer (Edif instruments, Italy) as mg/dl.
World Health Organization (WHO) criteria was used to define anemia as: Hgb concentration <13 g/dl for males and < 12 g/dl for females.43It was further classified into mild anemia (female: 11–11.9 g/dl; male: 11–12.9 g/dl), moderate anemia (8–10.9 g/dl) and severe anemia (< 8 g/dl).44Microcytic was defined as MCV < 80 fl, macrocytic: MCV >100 fl and hypochromic: MCHC value < 31 g/dl.29Serum creatinine values were considered as abnormal if values of serum creatinine analysis were > 1.5 mg/dl for males and > 1.3 mg/dl for females.45Good glycemic control: an average of four consecutive fasting blood glucose measurement was ≤ 130 mg/dl and Poor glycemic control: an average of four consecutive fasting blood glucose measurement was >130 mg/dl.46
Kidney function was estimated by using the simplified version of the Modification of Diet in Renal Disease (MDRD) study equation: 186 × SCr (mg/dl)−1.154×age (years)−0.203× 0.742 (if female) × 1.210 (as the study participants are black). Based on the result, it was classified as normal or increased estimated glomerular filtration rate (eGFR ≥ 90 mL/min/1.73 m2), mild renal impairment (eGFR 60–89.9 mL/min/1.73 m2), moderate and severe renal impairments (eGFR <60 mL/min/1.73 m2).47
To ensure good data quality, the questionnaire was pre-tested on 5% of the actual sample size on those people who did not participate in the study, training was given for data collectors, physical measurements were recorded three times, and close follow-up of the data collection process was carried out. Blood sample quality was also ensured during pre-analytical, analytical, and post-analytical stages by following the standard operating procedures.
Before entry, data were cleaned and checked; for any missing values. Then, it entered into Epi-data manager version 4.4.1.0 and was exported to SPSS version 22 statistical software for analysis. Then the data were processed by using descriptive analysis like frequency distribution, cross-tabulation, and summary measures. Multivariate logistic regression analysis (backward stepwise) was carried out for the selected variables with p-value < 0.25 in the bivariate logistic regression analysis and the corresponding adjusted odds ratios (AOR) with 95% confidence intervals (CI) were used to identify factors independently associated with anemia. P-value with < 0.05 was considered as statistically significant.
Results
A total of 249 T2DM patients, of which 128(51.4%) were females included in the study. Their ages ranged from 36 to 80 years with a mean (± SD) of 53.71 ± 10.41 years. More than half of the participants, 132 (53%) were aged 45 to 60 years. From the total of the respondents, 172 (69.1%) were married while 31(12.4%) were single, and 46(18.4%) were divorced and widowed. One hundred seventy-two (69.1%) of the participants were from urban areas. About 71(28.5%) of the respondents had a higher educational status. Regarding the employment status of the participants, 104 (41.8%) were a government employee and 45 (18.1%) employed at a private organization (Table 1).
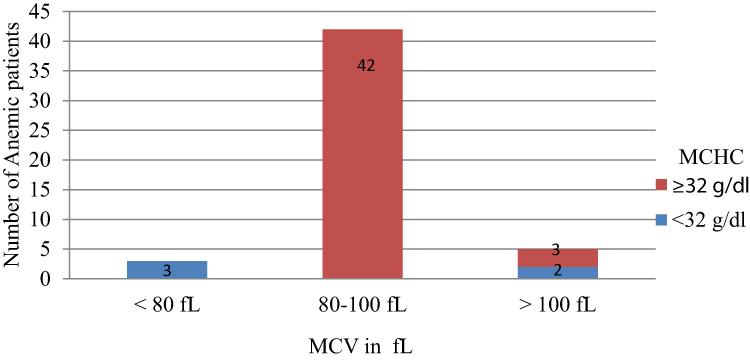
Table 1 Socio-Demographic Characteristics of T2DM Patients at Debre Berhan Referral Hospital, North-East Ethiopia, 2019 (n=249) |
The duration of DM was ranged from 8 months up to 24 years, with a mean (±SD) of 7.49±4.6 years. From the total of the respondents, 102 (41%) were with less than five years’ duration of DM, followed by 84 (33.7%) from five to ten years. The result of BMI of the patients at the time of study indicated that 162 (65.1%) and 76 (30.5%) of them had normal BMI (18.5–24.9 Kg/m2) and higher BMI (≥ 25 kg/m2), respectively. Seventy-eight (31.3%) of participants presented with documented records of at least one of the diabetes-related microvascular complications. Retinopathy 33 (13.3%) was the most prevalent complication followed by diabetic nephropathy 15 (6%), diabetes-related foot ulcer 14 (5.6%), neuropathy 6 (2.4%), and with more than one complications 10 (4%). Eighty-three (33.3%) of the participants were hypertensive, with 77 (30.9%) and 63 (25.3%) of participants SBP of ≥ 130 mmHg and DBP of ≥80 mmHg, respectively. The average of four consecutive fasting blood glucose levels (FBG) including FBG during study periods ranged between 101.25–264.25 mg/dl with a mean (± SD) of 147.90±35.21 mg/dl. More than half (54.2%) of the participants were presented with the level of poor glycemic control.
The majority (85.1%) of the participants had normal serum creatinine levels. The mean estimated GFR was, 95.63± 26.2 mL/min/1.73 m2. One hundred fifty-seven (63.1%) of the study participants had eGFR > 90 mL/min/1.73 m2whereas 43 (17.3%) of participants were found with eGFR of <60 mL/min/1.73 m2(Table 2).
Table 2 Clinical Characteristics of the Study Participants at Debre Berhan Referral Hospital, North-East Ethiopia, 2019 (n=249) |
The hemoglobin level of the participants was, from 9.4 g/dl to 17.5 g/dl, with a mean (± SD) of 14.32 ± 1.68 g/dl. The mean (± SD) of hemoglobin was 14.73 ± 1.53 g/dl and 13.93 ± 1.73 g/dl in male and female participants, respectively. The overall prevalence of anemia in the study participants was found to be 20.1% (95% CI = 15.3–25.3%); with 23 (19.01%) in males, and 27 (21.1%) in females. The mean (± SD) of Hb levels for males and females anemic patients were 12.2± 0.73 g/dl and 11.4±0.58 g/dl, respectively. Out of anemic T2DM patients, 42 (84%) and 8 (16%) had mild and moderate anemia, respectively. Severe anemia was not detected in this study. From these anemic patients, none of them was ever screened for anemia (Figure 1).
Figure 1 Prevalence and degree of anemia among type 2 DM patients; attending at Debre Berhan Referral Hospital, North-East Ethiopia, April 1 – May 30, 2019. |
The mean (± SD) of MCV was 92.5 ± 5.2 fL and 91.6 ± 4.9 fL in anemic and non-anemic patients, respectively. Likewise, the mean MCHC (± SD) was 33.9 ± 1.6 g/dl and 34.2 ± 1.6 g/dl in anemic and non-anemic patients, respectively. The differences in the distribution of both MCV and MCHC in non-anemic and anemic groups were not statistically significant (p>0.05).
The majority of anemic patients, 42 (84%) had MCV between 80–100 fL, and 45 (90%) of anemic patients had MCHC above 32 g/dl. Overall 42 (84%) of patients had normocytic normochromic, 3 (6%) had microcytic hypochromic, and 5 (10%) macrocytic anemia (Figure 2).
Figure 2 Distribution of MCV and MCHC of anemic type 2 DM patients; attending at Debre Berhan referral hospital, North-East Ethiopia, April 1- May 30, 2019. |
To evaluate the association of each independent variable with the occurrence of anemia, binary logistic regression was performed between the occurrence of anemia (dependent variable) and selected factors (independent variable). To identify the most significant determinant of anemia, factors that showed a p-value ≤ 0.25 in the bivariable analysis were a candidate to the multivariate logistic regression model. In multiple logistic regression analysis, patients with age >60 years (AOR=3.06, 95% CI: 1.32–7.11), poor glycemic control (AOR=2.95, 95% CI: 1.22–7.15), duration of DM >10 years (AOR=2.75, 95% CI: 1.17–6.48), diabetic complications (AOR=3.81, 95% CI: 1.65–8.81), eGFR 60–89.9 mL/min/1.73 m2(AOR=2.91, 95% CI: 1.15–7.37), eGFR <60 mL/min/1.73 m2(AOR=6.58, 95% CI: 2.42–17.93), were significantly associated with the occurrence of anemia (Table 3).
Table 3 Multi-Variable Logistic Regression of Variables Associated with Anemia Among T2DM Patients Attending at Debre Berhan Referral Hospital, North-East Ethiopia, 2019 (n=249) |
Discussion
In this institutional-based cross-sectional study, the prevalence of anemia and its associated factors among T2DM patients at Debre Berhan Referral Hospital, North-East Ethiopia, has been assessed. It was found that one out of five T2DM patients had anemia. It is also found that the prevalence of anemia was significantly associated with the different stages of renal function as evidenced by eGFR, age of the patients, duration of DM, level of glycemic control, and presence of diabetes-related complications.
In this finding, the total prevalence of anemia among T2DM patients was 20.1% (95% CI = 15.7–25.3%). This is in line with the study done in Australia (23.3%),48China (22.0%),26and, Iran (19.6%).24However, this finding was relatively higher than a study conducted by Rani et al, in India (12.3%),49and Kuwait (13%).50On the other hand, this finding was relatively lower than a cross-sectional study conducted in Brazil (34.24%),21Kuwait (29.7%),35Egypt (63%),33United Kingdom (59%),51Caribbean (46.5%),28Iran (30.4%),52Pakistan (63%),53Malaysia (31.7–39%),54,55and Fenote Selam Hospital (29.8%).32These differences might be due to differences in the geographical elevation above sea level, ethnicity, age of the study participants, duration of DM, and the level of development of the country since it affects the quality of health care delivery.6,8,33,43,56
The study tried to demonstrate the common morphological characteristics of anemia among T2DM patients. Normocytic normochromic blood picture was the most common morphological types of anemia found in this study. It is not surprising to see normocytic normochromic blood picture of anemia in this study, as various previous studies conducted in China,57Malaysia,54,55India,58,59and Iraq,60also revealed this situation. Since the participants of this study were DM patients, anemia of chronic disease is expected, which is normocytic normochromic in morphology.55,60–62In addition, normocytic normochromic anemia might suggest the significance of the renal origin of anemia in diabetic patients.6,7,63,64Considering the percentage of anemic patients with altered renal function in this study, the above explanation seems acceptable. Renal impairment leads to anemia though impaired production of erythropoietin by peritubular fibroblast of the kidney,65–67urinary erythropoietin losses,68reduced RBC life span due to uremic environment and the possible role of circulating uremic-induced prevention of erythropoiesis.69,70
However, this study deviates from another study conducted in India, which showed a higher rate of the microcytic hypochromic type of anemia.71The low prevalence of microcytic hypochromic anemia in this study could be explained by their residence and access to health care services. In this study, closer to three-fifth of the participants were from urban areas where health care services related to appropriate nutrition and a variety of nutrition accessible.
Although the study did not assess dietary status, it is unlikely that the anemia was due to nutritional deficiencies since the anemia was normocytic normochromic in the majority (84%) of patients. Regarding the levels of anemic status, the majority (84%) of the respondents had mild anemia; which is also common in anemia of chronic diseases like DM. This study is similar to the finding in Malaysia.54,55
In this study, there was a statistically significant relation between diabetes-related complications and the occurrence of anemia. As microvascular complications were frequently seen in uncontrolled and long-standing diabetes,53the odds ratio of developing anemia in patients with at least one diabetic complication were approximately four times more likely as compared with those without complication.
The study also showed that greater odds for the occurrence of anemia among T2DM patients with age > 60 years when compared with those of age ≤ 60 years. Consistent with the present finding, the increased odds ratio for developing anemia has also been found in the previous study conducted in California,56Australia,12China,57Israel,72Nigeria,25and Finote Selam hospital.32
In addition, the mean age of anemic participants (62.0±10.2) is significantly greater than the mean age of non-anemic participants (51.6±61.4). This result is in-line with a recent study in Kuwait (2018) which showed that old age to be associated with the higher prevalence of anemia in DM patients with the mean age of anemic and non-anemic patients were found to be 60.69 ± 0.198 years, 54.07 ± 0.121 years, respectively.35The study conducted in Korea,73and Australia12has also indicated that the prevalence of anemia increases with advancing age. This result was anticipated since aging is related to decreased hemoglobin levels and an increase of anemia irrespective of health status.74,75It also may be related to deficiencies of vitamins such as folate, bone marrow abnormality, and a higher number of co-morbidities, which are common at elderly.73
In this finding, the duration of DM is one of the factors associated with the presence of anemia. It was observed that a positive relationship between the duration of DM and anemia with a higher chance in patients with >10 years. Compared with patients with ≤ 10 years duration of DM, the odds ratio of developing anemia in individuals with ˃ 10 years was approximately three times. This finding is in agreement with the previous studies in Australia,12Korea,76India,49,77and Finote Selam.32The reason for this increased chance of anemia development with an increasing duration of DM may be due to the chronic effects of hyperglycemia. Diabetes-related chronic hyperglycemia can cause a chronic hypoxic milieu in the renal interstitium and disturbance of the interstitial organization or vascular architecture, atypical cell growth and collagen proliferation in tubular cells and peritubular fibroblasts, which cause impaired synthesis of erythropoietin by the peritubular fibroblasts.6,20In addition, in patients with prolonged hyperglycemic conditions, the erythrocyte precursors cells in the bone marrow might be exposed to prolonged direct glucose toxicity leading to disturbances in the erythrocyte production.35
This study indicated that the odds of developing anemia among respondents with poor glycemic control were three times more likely as compared with respondents with good glycemic control. This is comparable with findings in Nigeria,78India,58Pakistan30and Kuwait.35Because erythropoietin synthesis and release are controlled in part by the autonomic nervous system,22,25and diabetic autonomic neuropathy is common in a condition of poor glycemic control,79,80the results propose that erythropoietin synthesis could be prematurely inhibited in patients with poor glycemic control. In addition, in participants with poorly controlled DM, precursors of erythrocyte in the bone marrow might also be exposed to prolonged direct glucose toxic effects or mature red blood cells can be affected by oxidative stress causing disturbances in the red blood cells function.9
Additional factors which have been implicated in the risk of anemia associated with hyperglycemia include; systemic inflammatory damage to renal architecture and consequent formation of AGEs and its effects on bone marrow.10,12,20,22,34,81–83These circumstances may be provoked in poorly controlled diabetes than in controlled diabetes.
This finding showed a considerable relationship between anemia and a decline in eGFR. Out of 50 study participants who were anemic, 36(72%) showed <90 eGFR mL/min/1.73 m2and subsequently presented with normochromic normocytic anemia, which is considered to be an indication that the cause of anemia may be due to renal dysfunction. The odds of being anemia in participants with T2DM and mild renal impairments (eGFR 60–89 mL/min/ 1.73 m2) were roughly three times as compared to participants with normal renal function (≥90 mL/min/1.73 m2). The odds ratio for developing anemia among participants with T2DM and moderate renal impairment (eGFR < 60 mL/min/ 1.73 m2) was also approximately seven times as participants with normal renal function (≥90 mL/min/ 1.73 m2). The study conducted in Australia,7Finote Selam,32Greece,84and Cameroon,31supported these findings.
Anemia is a well-known complication of diabetes-related chronic kidney disease (CKD) and related to the degree of renal impairment, mainly due to impaired production of erythropoietin by peritubular fibroblast of the kidney.84The finding in this study further supports this principle, as the results showed a gradually increase in the prevalence of anemia with a progressive reduction in renal function. There are many potential mechanisms, by which anemia can exist in patients with reduced renal function tests. Damage to erythropoietin producing cells through either fibrosis or chronic inflammatory activities; tubulointerstitial changes; and autonomic neuropathy, which prevents anemia detection by peritubular fibroblasts of the kidney are the possible mechanism.11,85
Even though this study has not attempted to find the cause of anemia, those patients at the highest risk could be recognized by the presence of renal impairment, identified as impaired renal function <90 mL/min/1.73 m2in patients with DM. This indicates that the main cause of anemia may be of the renal origin. Additionally, the occurrences of anemia in patients with DM should direct health professionals to examine the likelihood of renal involvements.7
The strength of this study is that it is one of the few studies in developing countries where chronic diseases like DM with associated complications are becoming more common. The study has its limitation as lacks of age and sex-matched control groups; due to the nature of the study design, cause/effect relationship not identified; the dietary pattern was not assessed; Hb level did not adjusted for altitude; as well as the level of glycemic status was assessed by FBG rather than HbA1c. The other limitation of this study is that the cut off point for anemia used in this study is not validated among adult populations of Ethiopian origin.
Conclusion
One out of five T2DM patients had anemia, including among those with normal renal function evidenced by eGFR. Most of the anemic patients had a mild type of anemia. Morphologically, the predominant type of anemia was normocytic normochromic anemia.
Poor glycemic control, decreased eGFR, diabetes-related complications, duration of DM >10 years, and age >60 years were significantly associated. The results suggest the necessity for incorporating regular screening for anemia in all T2DM patients mainly for patients with these identified risk factors to facilitate early detection and management of anemia among T2DM and consequently improve the overall care of these patients.
